Embarking on a research journey, we often find ourselves at the crossroads of choosing the appropriate methodology to map out evidence and discern gaps within a particular domain. Understanding the scoping review process, benefits, and protocol is pivotal for us, as it provides a holistic view of the evidence available. Scoping reviews represent a fundamental framework that guides researchers, clinicians, and policymakers within various fields to synthesize information effectively. The initial phase of a scoping review, known as the protocol development, is an integral component that defines the direction and depth of the review.
Scoping Review Usage Guide: Best Practices
A comprehensive guide to conducting effective scoping reviews
1. Introduction to Scoping Reviews
A scoping review is a systematic approach to mapping evidence across an area of research. It is particularly useful for:
- Examining emerging evidence when it is still unclear what specific questions can be addressed by a systematic review
- Mapping key concepts, types of evidence, and gaps in research
- Clarifying working definitions and conceptual boundaries of a topic
2. When to Choose a Scoping Review
Appropriate Scenarios:
- Examining the extent, range, and nature of research activity
- Determining the value of undertaking a systematic review
- Summarizing and disseminating research findings
- Identifying research gaps in existing literature
Key Differences from Systematic Reviews:
| Aspect | Scoping Review | Systematic Review |
|---|---|---|
| Purpose | Maps evidence landscape | Answers specific question |
| Question | Broader in scope | Focused, precise |
| Quality Assessment | Optional | Mandatory |
| Synthesis | Typically narrative | Often meta-analysis |
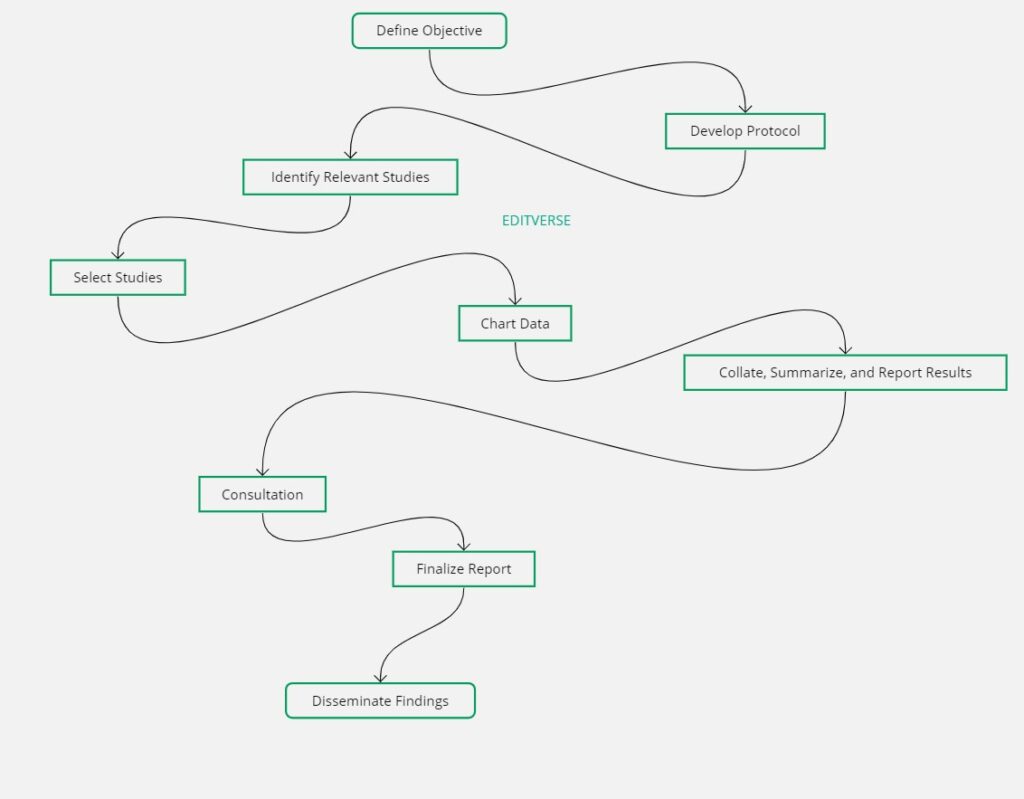
3. Step-by-Step Process
Stage 1: Planning
Identify Research Question
- Use PCC framework (Population, Concept, Context)
- Keep questions broad but with clear scope
Develop Protocol
- Register protocol (e.g., OSF, Figshare)
- Define inclusion/exclusion criteria
- Document search strategy
Stage 2: Searching
Search Strategy Development
- Identify key terms and concepts
- Consult librarian if possible
- Include multiple databases
4. Timeline Planning
| Stage | Time Required |
|---|---|
| Protocol Development | 2-4 weeks |
| Literature Search | 2-3 weeks |
| Screening | 4-8 weeks |
| Data Extraction | 4-6 weeks |
| Analysis & Writing | 6-8 weeks |
5. Best Practices Checklist
✅ Do:
- Clearly define boundaries
- Involve multiple reviewers
- Document all decisions
- Acknowledge limitations
❌ Don’t:
- Make the scope too broad
- Skip protocol registration
- Conduct quality assessment without justification
- Draw conclusive recommendations
Our commitment to formulating a robust scoping review protocol embodies our endeavor to ensure that the review process is transparent, and the findings genuinely benefit the scientific community and stakeholders. By meticulously planning and outlining the scoping review protocol, we establish a clear pathway that not only informs our conduct throughout the study but also reinforces the integrity of the information we present to our audience.
Scoping Review Usage Guide: Best Practices
Contact for Support: su*****@*******se.com
Evidence-Based Statistics:
- Average number of included studies: 1-2600 (mean of 118 studies)
- Recommended minimum: Two independent reviewers for data extraction
- Duration varies significantly based on scope and team size
- Systematic approach with predefined protocols required
1. When to Choose a Scoping Review
“Choose scoping reviews to examine the extent, range, and nature of evidence on a topic” – Arksey & O’Malley Framework
- Ideal Scenarios:
- Emerging or broad topics
- Complex or heterogeneous evidence
- Need to identify research gaps
- Clarifying key concepts/definitions
- Not Suitable For:
- Clinical effectiveness questions
- Treatment comparisons
- Quality assessment of evidence
2. Framework Selection
- Popular Frameworks:
- Arksey & O’Malley Framework (2005)
- JBI Methodology (2015)
- PRISMA-ScR Guidelines (2018)
- Key Steps:
- Research question formulation
- Relevant studies identification
- Study selection
- Data charting
- Results collation and reporting
3. Protocol Development
- Essential Components:
- Background and rationale
- Review objectives
- Inclusion criteria (PCC framework)
- Search strategy
- Data extraction tools
- Registration Options:
- OSF Registries
- Figshare
- Research Registry
4. Search Strategy Development
- Key Databases:
- MEDLINE/PubMed
- Embase
- Web of Science
- Grey Literature sources
- Search Components:
- Population terms
- Concept terms
- Context terms
- Study design filters (optional)
5. Data Charting Process
- Essential Data Fields:
- Author(s) and year
- Study location/setting
- Population characteristics
- Methodology
- Key findings
- Research gaps identified
- Best Practices:
- Use at least two independent reviewers
- Develop standardized data extraction forms
- Conduct pilot testing of forms
- Regular team meetings for consistency
6. Results Presentation
- Visual Representations:
- PRISMA flow diagram
- Evidence maps
- Conceptual frameworks
- Frequency tables
- Narrative Synthesis:
- Thematic analysis
- Chronological development
- Geographical distribution
Need Additional Support?
Contact our expert team for guidance and assistance:
- Email: su*****@*******se.com
- Response Time: Within 24-48 hours
- Available for consultation on:
- Protocol development
- Search strategy refinement
- Data charting templates
- Results presentation
For further assistance, email: su*****@*******se.com
Key Takeaways
- Scoping reviews provide a comprehensive mapping of evidence, crucial for researchers across various disciplines.
- The implementation of a scoping review protocol is the foundational step that ensures clarity and transparency.
- Understanding the scoping review process aids in identifying gaps and paves the way for future research.
- Methodical structuring of scoping review benefits both healthcare decision-making and policy formulation.
- Our approach to guiding scoping review protocol development is designed to enhance the applicability and effectiveness of the review’s outcome.
Understanding Scoping Reviews in Evidence Synthesis
Our exploration of evidence within the fields of health care and beyond is often guided by a defined methodology involving scoping reviews. A scoping review definition delineates it as a research tool employed to map out existing literature in a systematic, inclusive manner without the analytical comparisons that are hallmark of systematic reviews. Instead, scoping reviews draw from a breadth of evidence to outline the landscape of knowledge and inform stakeholders on the state of research within specific domains.
To enhance the application of scoping reviews, we work in accordance with established scoping review guidelines, which direct us in assembling a protocol that is rigorous, transparent, and replicable. These guidelines are instrumental in clarifying our objectives, refining the research questions, and delineating the criteria for inclusion and exclusion of studies, in addition to prescribing the methodology for data extraction and synthesis.
Gleaning from scoping review examples, we observe a commonality in their purpose—to provide a wide-angle view of the literature available, draw connections among different study results, and reveal patterns and gaps in research. Our protocols thus follow a structured process to encapsulate the essence of the data collated, offering a panoramic view that informs further inquiry and policy.
| Scoping Review Step | Description | Purpose |
|---|---|---|
| Defining the Research Question | Identifying the main objectives and the extent of the scoping review. | To ensure focused retrieval of relevant literature. |
| Developing Protocol | Outlining procedures for search strategy, inclusion, extraction, and synthesis of data. | To maintain methodological transparency and replicability. |
| Literature Search | Extensive database search to gather pertinent studies aligning with the defined question. | To compile a comprehensive dataset representing the current state-of-research. |
| Selection of Studies | Applying the pre-defined criteria to filter the literature. | To ensure relevance and scope coverage of selected articles. |
| Data Extraction | Systematically drawing information from the chosen studies. | To distill essential elements that inform the evidence map. |
| Data Synthesis | Integrating the data to articulate key themes, concepts, and knowledge gaps. | To provide structured insights and direction for future research. |
In our dedication to the scientific community’s advancement, we assiduously abide by these meticulously crafted procedures within our scoping review endeavors. Through this, we contribute to a nascent body of evidence, poised for strategic expansion, and ultimately, to the betterment of practices and policies influenced by the research we synthesize and report.
Scoping Review When to Use: Indications and Appropriateness
When embarking on a journey of evidence synthesis, it’s crucial for us to discern whether a scoping review is the most fitting approach for our research objectives. Scoping reviews are highly beneficial when our aim is to explore the breadth of evidence on a given topic, especially when the field is complex or has not been comprehensively reviewed.
It’s imperative to understand the scoping review when to use not only to guarantee the efficacy of research efforts but also to leverage the scoping review benefits. Such an understanding will ultimately shape the outcomes of our research, influencing policy and practice in meaningful ways.
As researchers in Japan, we frequently navigate through volumes of literature across disciplines, determining the necessity and utility of a scoping review. Our methodological rigor and strategic foresight support us in choosing the review methodology that will best serve our research pursuits and the communities that rely on our findings.

Clarifying Scoping Review Definitions and Objectives
Defining the scoping review’s scope and objectives is the first step in our evidence synthesis journey. A scoping review’s primary objective is not to evaluate the quality of evidence but to map it. We aim to identify research trends, gaps, and emerging themes within a certain domain. This is paramount when investigating areas where research is burgeoning or when the evidence base is vast yet disparate.
Our understanding of scoping review methodology drives the development of review protocols that go beyond mere data aggregation. Instead, we aspire to produce a comprehensive overview that both informs and instructs future concentrated reviews.
Scoping Versus Systematic Review: Making the Right Choice
In contrast to a systematic review, which seeks to answer a specific research question through rigorous evaluation and synthesis of evidence, a scoping review casts a wider net. The decision to choose a scoping review vs systematic review hinges on our goal: whether we are laying the groundwork for further study or looking to methodically summarize existing research on clinical interventions.
We consider the scoping review particularly when our aim is to clarify concepts, examine scopes of practice, or prepare for a subsequent, more targeted systematic review. The flexibility of the scoping review methodology is key in addressing broad questions, laying the foundations for focused investigations that may arise.
Identifying Knowledge Gaps and Emerging Evidence Through Scoping Reviews
Through our scoping reviews, we endeavor to uncover knowledge gaps and provide a clearer picture of untapped research opportunities. Our methodical approach involves cataloging and characterizing the evidence, setting the stage for future investigations. It’s this careful and conscientious process that makes the scoping review an invaluable tool in our evidence synthesis arsenal.
Amid emerging evidence, the scoping review is our methodology of choice for navigating through new terrains of research, offering substantial benefits in conceptual clarity and strategic research planning. As we undertake each review, we become more adept at utilizing this versatile approach to serve as a compass in our evolving landscape of healthcare research and policy development.
Scoping Review Methodology: Steps to Ensure Thoroughness and Transparency
As passionate researchers, we are committed to ensuring that each step in our scoping review process adheres to the principles of thoroughness and transparency. This dedication is reflected in our approach to developing a scoping review methodology that not only satisfies the curiosity of our peers in Japan but also contributes to the global compendium of knowledge.
Our carefully devised scoping review protocol underpins our every action, setting out an unequivocal roadmap for the scoping review process. It is our blueprint for identifying relevant literature, summarizing findings, and recognizing the often nuanced gaps in existing research. Let us walk you through the fundamental steps that define our methodology:
| Step | Activity | Rationale |
|---|---|---|
| 1. Establishing a clear review question | Determining the aim and breadth of the scoping review to guide the literature search. | A precise question ensures the review remains focused and relevant. |
| 2. Formulating inclusion and exclusion criteria | Defining the parameters that determine which studies will be considered for inclusion. | Clear criteria are critical for both the integrity of the scoping review and the replicability of the process. |
| 3. Conducting a comprehensive literature search | Searching various databases and sources to gather a broad range of studies. | To capture the full scope of existing evidence relevant to the research question. |
| 4. Selecting appropriate studies | Applying the criteria to filter the literature collected during the search phase. | To ensure the selected studies align with the objectives of the review and the research question. |
| 5. Extracting and charting data | Systematically pulling relevant information from each study. | Accurate data extraction provides the foundation for meaningful analysis and synthesis. |
| 6. Synthesizing findings | Integrating the collected data to highlight patterns, themes, and gaps in the research landscape. | An insightful synthesis offers value to the academic and clinical communities by elucidating the state of current knowledge. |
Faithful to our rigorous protocols, we report our methods and findings with precision, allowing our work to be testable by fellow academics and practitioners. This is why we also abide by the recognized reporting standard, PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), specifically adapted for scoping reviews to ensure the quality and credibility of our endeavors.

To us, these methodological underpinnings are not mere formalities but are integral to the integrity of our work. By applying a rigorously structured approach to scoping review methodology, we pave the way forward for subsequent evidence synthesis, grounded in the robust and transparent presentation of our findings. It is our hope that through these meticulous practices, we impart lasting contributions to the repository of global health knowledge and support the refinement of evidence-based practices.
Conclusion
In an era where the landscape of research methodologies continues to expand, we’ve seen first-hand the pivotal role scoping reviews play in evidence synthesis. Throughout our discussion, we have delved into the steps and guidelines of the scoping review methodology, emphasizing how it diverges from systematic reviews to serve a unique purpose. In essence, scoping review guidelines construct a solid framework for our inquiries, allowing us to depict a comprehensive landscape of existing knowledge within broad research fields.
A cornerstone of our research strategy, scoping reviews facilitate the identification of gaps, broaden our understanding of subject matter, and lay the groundwork for future analysis or systematic reviews. Moreover, by showcasing profound scoping review examples, we illustrate the adaptability and depth this approach affords researchers and policymakers alike in Japan and beyond. Our experiences suggest that when precisely and appropriately applied, scoping reviews greatly enhance the collective understanding and application of evidence, thereby influencing healthcare outcomes and policy development.
As guardians of research integrity, we are compelled to advocate for transparency and diligence in the application of any evidence synthesis methodology. Thus, we posit that scoping reviews should be deployed with clear intent and firm grounding in established criteria. When employed under such prerequisites, the benefits of scoping reviews are manifold, rendering them an indispensable tool in the pursuit of well-informed, evidence-based practice.
FAQ
What is a scoping review?
A scoping review is a form of knowledge synthesis that aims to map key concepts, available evidence, and research gaps within a defined area. It is an exploratory approach that provides a broad overview of a topic without performing a detailed assessment of study quality or risk of bias. Scoping reviews are typically used to clarify definitions and concepts and to identify key characteristics or factors related to a concept.
When should a scoping review be used?
A scoping review should be used when the purpose of the research is to identify and map out the breadth of a particular field, to clarify key concepts and definitions within that field, or to identify knowledge gaps that could guide future research. It is particularly useful for emerging topics or when the literature on a subject is vast and complex.
What are the benefits of conducting a scoping review?
Conducting a scoping review offers several benefits, including the ability to provide a structured overview of the literature, to inform policy and practice, to identify research gaps, and to serve as a precursor to a more detailed systematic review. It also helps to consolidate evidence on topics where there is a diverse range of study designs or where a systematic review may not be feasible due to the broad nature of the research question.
How does a scoping review differ from a systematic review?
Scoping reviews differ from systematic reviews mainly in their objectives and methods. Scoping reviews aim to provide an overview of existing evidence without synthesizing results, while systematic reviews seek to consolidate findings and often assess the risk of bias in order to answer specific clinical or policy-driven questions. Unlike systematic reviews, scoping reviews do not typically include meta-analysis due to their broader scope and the exploratory nature of the research questions they address.
What should a scoping review protocol include?
A robust scoping review protocol should outline the rationale, objectives, and research question(s) of the review. It should provide clear inclusion and exclusion criteria for studies, describe the method for searching and selecting relevant literature, and detail the approach for extracting and charting the data. Additionally, it should describe the process for synthesizing the findings and specify any stakeholder involvement.
What are some examples of when a scoping review is appropriately used?
Examples of appropriate use cases for scoping reviews include examining the extent of research on a given topic, identifying types of available evidence in emerging areas of research, exploring complex or heterogeneous topics where study designs may differ, and clarifying key concepts or definitions in a field.
What are the guidelines for reporting a scoping review?
Guidelines for reporting a scoping review are outlined in the PRISMA Extension for Scoping Reviews (PRISMA-ScR). These guidelines promote transparency and uniformity in the reporting of scoping reviews, ensuring that they include essential items such as the rationale, eligibility criteria, information sources, search strategy, data extraction process, and synthesis of results.
How is the scoping review process structured?
The scoping review process is typically structured into several key stages: (1) identifying the research question, (2) developing and following a protocol, (3) conducting a comprehensive literature search, (4) selecting studies based on clear inclusion and exclusion criteria, (5) charting the data by extracting key information, and (6) collating, summarizing, and reporting the results. There is often an iterative element to the process, with the potential for refinement at any stage.