Imagine you’re on a voyage, navigating the complex biology of the human body. Picture the cellular machinery where molecules communicate in a language unique to them—signals passing back and forth like ships on a bustling harbor. Now, the mTOR pathway stands as the lighthouse within this intricate network, guiding the processes that dictate cell growth and survival. Yet, this lighthouse can sometimes lead astray. In the realm of oncology, the hyperactivation of the PI3K/AKT/mTOR pathway is akin to a beacon gone rogue, derailing the normal course of events and leading to the emergence of cancer’s daunting adversary: drug resistance1.
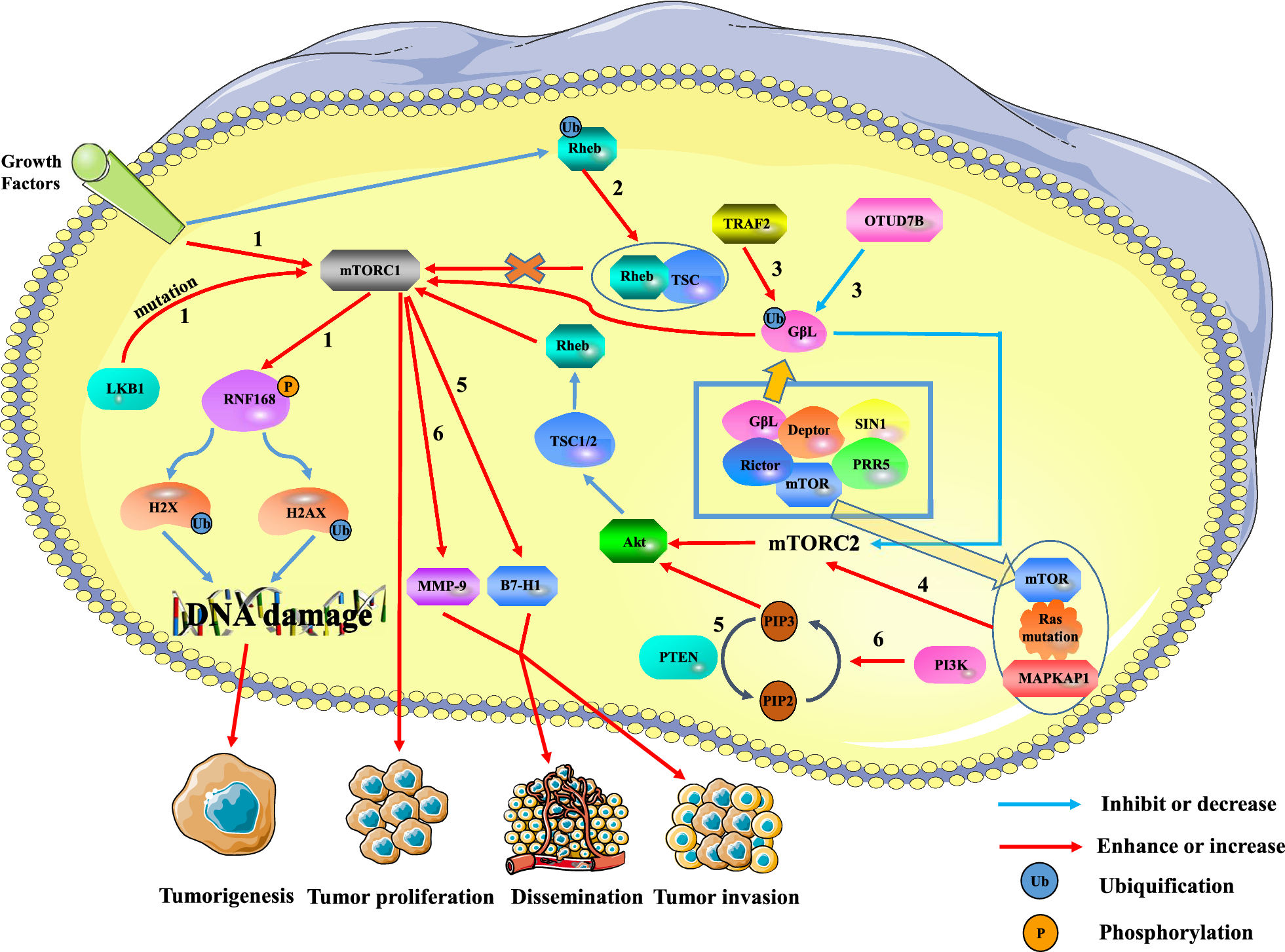
The relationship between mTOR and tumors is intricate. Overactivation of mTORC1 can drive tumor formation, proliferation, and metastasis, while mTORC2 can influence the activity of mTORC1 through the mTORC2/AKT/TSC/Rheb pathway. There are several pathways through which mTOR impacts tumor formation and progression. Pathway 1 involves extracellular growth signals and intracellular LKB1 mutations activating mTORC1, leading to reduced DNA repair and tumor formation. Pathway 2 suggests that the ubiquitination of Rheb reduces mTORC1 activity, inhibiting tumor growth. Pathway 3 demonstrates that TRAF2 and Otud7B regulate mTORC1/2 activity by modulating the ubiquitination level of G beta L of mTORC2. Pathway 4 indicates that mutated Ras promotes mTORC2 expression, leading to tumor proliferation. Pathway 5 highlights how the deletion of the PTEN gene increases tumor progression and invasion. Pathway 6 involves the PI3K/PTEN/AKT/mTOR pathway in the invasion and metastasis of liver cancer by up-regulating MMP-9. [source: Zou, Z., Tao, T., Li, H. et al. mTOR signaling pathway and mTOR inhibitors in cancer: progress and challenges. Cell Biosci 10, 31 (2020). https://doi.org/10.1186/s13578-020-00396-1]
Your body’s natural mechanisms, including therapies like chemotherapy and targeted therapy, initially set sail to suppress the tumultuous waves of breast cancer, aiming to reduce the possibility of disease recurrence. However, as with any seasoned sea, the waters change, and many breast cancer patients face the storm of acquired resistance to these standard treatments1. Clinical trials become the compass points by which we navigate this challenge, testing various drugs targeting the notorious PI3K/AKT/mTOR pathway to overcome this resistance, especially given breast cancer’s notoriety as the leading cause of cancer mortality among women globally1.
Key Takeaways
- Understanding the mTOR pathway’s role in cell growth and drug resistance is crucial for tackling cancer.
- Standard cancer treatments are often thwarted by acquired resistance, necessitating new approaches.
- Clinical trials are pivotal in exploring targeted therapy options for breast cancer patients.
- The nuanced nature of breast cancer requires treatment strategies tailored to its molecular subtypes.
- Integrating drugs that inhibit the mTOR pathway can potentially lead to better patient outcomes.
- Knowledge of the PI3K/AKT/mTOR pathway’s contribution to resistance helps inform future cancer treatments.
Understanding the mTOR Pathway in Cancer Research
The mTOR pathway, a cornerstone in cancer research, has been extensively studied in relation to tumor progression and oncogenesis. This pathway, playing a pivotal role in cell growth and survival, has profound implications on the development and treatment of various cancers.
The Role in Tumor Progression and Drug Resistance
Research has demonstrated that activation of the PI3K/AKT/mTOR pathway is a critical player in the establishment and advancement of many cancers, linking molecular mechanisms to cellular behaviors that encourage cancer progression2. This pathway’s involvement in intrinsic and acquired drug resistance has made it a significant target for cancer therapeutics. The prevalence of mutations in the PI3K/AKT/mTOR pathway, especially in cancers like breast, prostate, and gastric, underline its crucial role in promoting cellular resistance to existing therapies2. Studies show that by targeting the PI3K/AKT/mTOR signaling pathway, reduction in tumor growth has been observed in colon cancer stem cells3.
Key Components of mTOR Signaling in Oncogenesis
Pivotal to the mTOR signaling pathway are components like PIK3CA gene mutations and PTEN alterations, which are frequently implicated in the dysregulation of signaling pathways2. The activation of these components leads to oncogenesis by enhancing the mTOR signaling, emphasizing the pathway’s centrality in tumor biology2. Recent studies suggest that targeting specific elements like PTEN, PI3K, or AKT can potentially lead to breakthroughs in cancer treatment, offering new avenues in therapeutic strategies3.
In conclusion, understanding the molecular mechanisms and key components of the mTOR pathway provides critical insights into its function in both oncogenesis and the resistance mechanisms of tumors. As research progresses, targeting the mTOR pathway in cancer therapy continues to hold promise for developing more effective anticancer treatments.
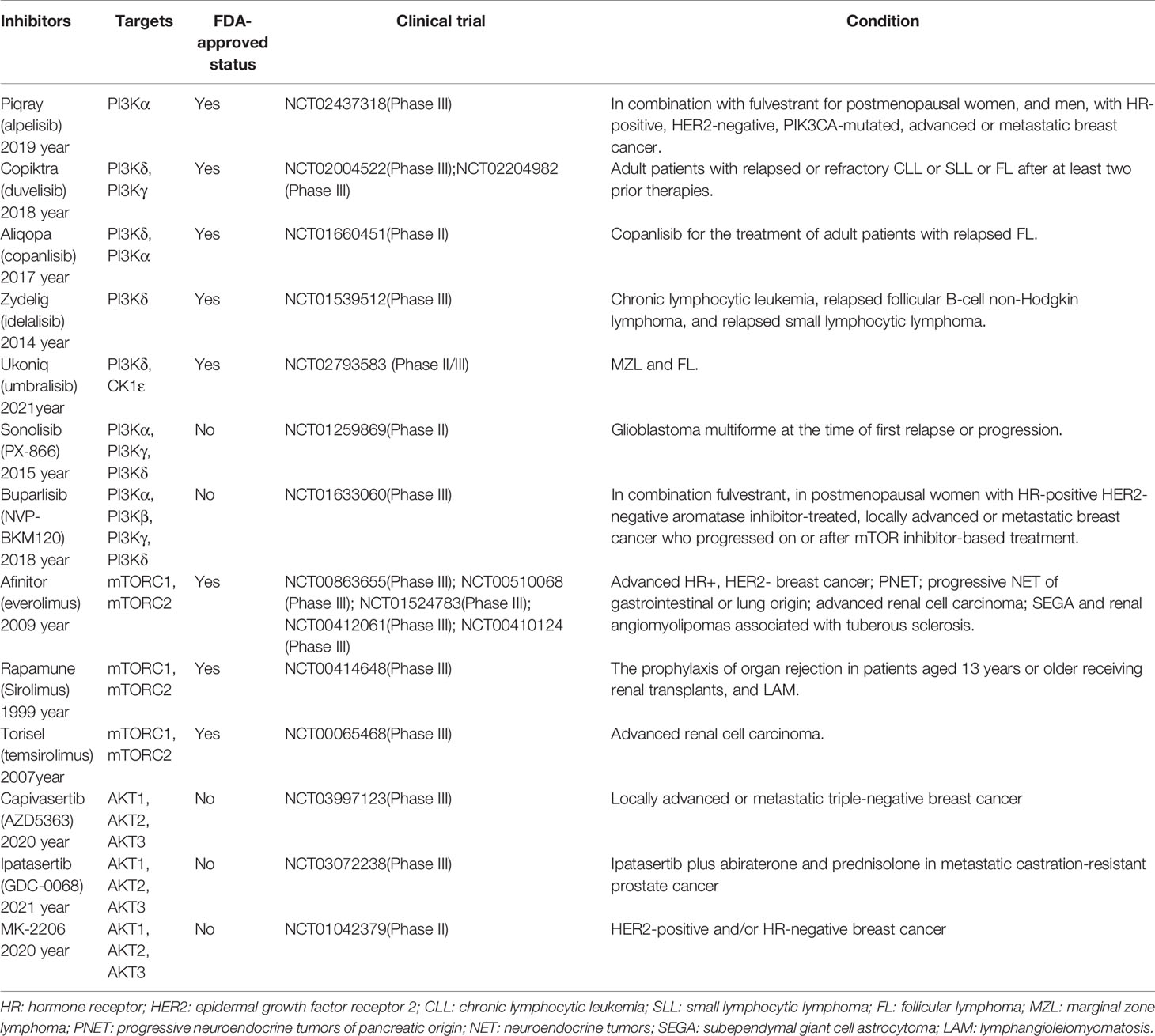
PI3K/AKT/mTOR pathway inhibitors.
Discovering Biomarkers: The Promise of Precision Medicine in Oncology
The intersection of precision medicine and biomarker research is revolutionizing treatment strategies in oncology, particularly within the realm of molecular oncology. Identifying specific biomarkers in cancer patients allows for the tailoring of treatments that are not only effective but also minimize unnecessary side effects.
Advances in genomic technologies have spurred significant breakthroughs in biomarker identification. The completion of the human genome project in 2003 marked a historic milestone, setting the stage for targeted genetic testing that supports the development of precision medicine4. Furthermore, the Cancer Genome Atlas Network’s molecular profiling of human breast tumors has provided deep insights into cancer-specific biomarkers, enhancing targeted therapeutic approaches5.
The ability to profile molecular characteristics of tumors enables clinicians to predict patient responses to specific drugs. For instance, studies like the MOSCATO 01 trial demonstrated that high-throughput genomic testing could significantly influence clinical outcomes in hard-to-treat advanced cancers by identifying actionable biomarkers6. Similarly, the Princess Margaret IMPACT/COMPACT trial showed that molecular profiling of solid tumors could lead to personalized medicine strategies that improve patient prognosis6.
The broader adoption of precision medicine has also been catalyzed by governmental initiatives, such as the launch of the precision medicine initiative by Barack Obama in 2015, aimed at revolutionizing how we improve health and treat disease4. This initiative emphasizes the importance of biomarker research in developing treatment strategies that are precisely targeted to individual genetic profiles.
In addition to genetic data, other forms of biomarkers such as proteomics and metabolomics are gaining prominence. These technologies contribute to a more comprehensive understanding of disease mechanisms, further enabling the personalized treatment of cancer4. Tumor mutational burden, for example, has been identified as a critical biomarker for predicting responses to immunotherapies in various cancers, emphasizing the growing role of comprehensive molecular profiling in treatment strategy development6.
Your engagement with cutting-edge precision medicine strategies is a pivotal factor in advancing cancer treatment. By staying informed about the latest developments in biomarker research, you contribute to the transformative changes shaping the future of oncology.
mTOR Pathway and its Impact on Targeted Cancer Therapies
The mTOR pathway, a pivotal component of oncological research, significantly influences the efficacy of targeted therapies in cancer treatment. By regulating signal transduction and exploring molecular mechanisms, researchers are developing interventions that directly impact cancer cell behavior.
Signal Transduction and Molecular Mechanisms of Targeted Therapy
Targeted therapy, focusing on the mTOR pathway in oncology, disrupts the complex sequence of signal transduction essential for cancer cell survival and proliferation. The introduction of specific inhibitors aims to dismantle the pathway at critical junctures, effectively halting cancer progression. For instance, studies show a 100% targeting of the PI3K/AKT/mTOR signaling pathway in recent breast cancer research, acknowledging its central role in future therapeutic strategies2.
Furthermore, the global prevalence of PI3K/AKT/mTOR pathway mutations across various cancers underscores the universal potential of targeted therapy in oncology2. The sensitivity of melanoma patients to pathway inhibitors, observed at 100%, also highlights the pivotal role of genetic factors in therapeutic outcomes2.
Challenges of Drug Resistance in Targeted Therapies
Despite advances, drug resistance remains a critical challenge, often manifesting through alternate signaling pathways or genetic mutations that bypass the targeted therapy. For example, the prevalence of PIK3CA mutations in breast cancer, detected in 22% of cases, correlates with variable treatment responses and necessitates ongoing adjustments to therapeutic protocols2.
In response to such challenges, oncologists and researchers emphasize a comprehensive understanding of molecular mechanisms, as mutations like AKT1(E17K) in breast cancer, present in 3% of samples, could guide the development of more refined, targeted treatments2.
| Cancer Type | Percentage of PI3K/AKT/mTOR Pathway Targeting |
|---|---|
| Breast Cancer | 100% |
| Gastrointestinal Stromal Tumors | 100% |
| Head and Neck Cancer | 34% (Pathway Mutations) |
| Melanoma (Sensitivity to Inhibitors) | 100% |
This data not only reflects the critical engagement with the mTOR pathway in oncology but also points to the targeted areas where resistance mechanisms may evolve, requiring continual research and adaptation of strategies to maintain the effectiveness of cancer therapies.
mTOR Pathway in Oncology: Clinical Trials and Drug Resistance
Understanding the complexities of the mTOR pathway in oncology is critical for advancing cancer treatment and addressing resistance mechanisms. Clinical trials targeting this pathway are particularly focused on addressing the challenge of acquired resistance, often observed in metastatic breast cancer, by integrating mTOR inhibitors alongside other therapeutic agents to improve patient outcomes.
Recent clinical trials have underscored the significance of the PI3K/AKT/mTOR pathway in breast cancer treatment, where resistance to standard chemotherapy and targeted therapy remains a considerable challenge. Drug resistance, particularly in aggressive cancer forms, is attributed to mutations in the PIK3CA gene, which lead to enhanced PI3K activation and subsequent resistance to anticancer therapies1. In response to these challenges, several drugs targeting the PI3K/AKT/mTOR pathway are under clinical examination, aiming to derail the cancer progression mechanism effectively1.
Moreover, FDA-approved PI3K inhibitors like alpelisib, idelalisib, and copanlisib are actively being utilized in treatments, signaling a shift towards targeted therapeutic approaches in oncology1. Notably, clinical trials have revealed the potential of using these inhibitors in combination with other medications to counteract the robust resistance mechanisms and prolong the efficacy of the treatment modalities.
Data derived from various clinical studies revealed that disruptions in the AKT gene could result in oncogenic activation, which plays a crucial role in how cancers develop and overcome current therapeutic strategies2. Studies span across multiple cancer types, including not just breast but also prostate, gastric, and lung cancers, highlighting the erratic and widespread impact of mutations within the mTOR pathway2.
The ongoing focus of clinical trials and research on the mTOR pathway is not only refining our understanding of cancer treatment but is also steering the advent of novel interventions. These strategies are particularly aimed at surmounting the intricacies of drug resistance, offering hope for more durable and effective solutions in cancer care. As we continue to explore and understand this pathway, the potential to develop transformative cancer treatments becomes increasingly attainable.
Drug Resistance Mechanisms and the mTOR Pathway
As cancer treatments evolve, the mTOR pathway has emerged as both a pivotal axis of resistance mechanisms and a promising target for therapeutic intervention. Understanding how drug resistance develops and persists in many forms of cancer—specifically through the mTOR pathway—can guide the development of more effective treatment strategies.143>p>
Overcoming Mechanisms of Resistance with Novel Therapeutic Strategies
Drug resistance often evolves due to the hyperactivation of the mTOR pathway, particularly marked by the frequent mutations in the PIK3CA gene, a phenomenon seamlessly connected to the progression of many cancers, including breast cancer1. Innovative therapeutic strategies, such as combination therapies utilizing PI3K inhibitors like alpelisib and idelalisib, have shown potential in clinical trials, particularly for specific breast cancer subtypes harboring these mutations1. Novel therapeutic approaches aim at meticulously disrupting compensatory signaling pathways or directly inhibiting downstream effectors of mTOR to reinstate drug efficacy7.
Signaling Pathways and their Relation to Drug Efficacy
Enhancing the efficacy of cancer drugs hinges on the profound understanding of signaling pathways involved in drug resistance mechanisms. The mTOR pathway interacts with an array of signaling cascades, including PI3K/AKT, promoting cellular proliferation, growth, and survival. This invariably leads to over-activation in certain tumors, circumventing the inhibitory effects of many targeted cancer therapies7. Strategic inhibition of these pathway interactions, therefore, holds the key to bolstering the effectiveness of mTOR-targeting drugs. Investment in research that maps out the intricacies of these pathways can inform the development of more robust therapeutic strategies that prevent the activation of alternate signaling routes, helping to manage or even reverse drug resistance mechanisms17.
| Drug Type | Example | Function | Used for Cancer Type |
|---|---|---|---|
| PI3K Inhibitors | Alpelisib, Idelalisib | Target PI3K/AKT/mTOR pathway | Breast Cancer, Various Cancer Types |
| mTOR Inhibitors | Everolimus | Inhibits mTORC1 leading to decreased cell proliferation | HR+/HER2- Breast Cancer |
| AKT Inhibitors | Taselisib | Distorts AKT signaling to decrease tumor growth | HR+/HER2- Breast Cancer |
Therapeutic Strategies Targeting the mTOR Pathway in Cancer
The relentless pursuit to refine cancer treatments has driven the focus towards therapeutic strategies that harness mTOR inhibition. This approach is pivotal in developing therapies that not only inhibit tumor growth but also counteract the intricate mechanisms of drug resistance.
Combination Therapies to Overcome Drug Resistance
In the era of personalized medicine, combination therapies have emerged as a cornerstone in cancer treatment, particularly through the inclusion of mTOR inhibitors. By targeting the mTOR signaling pathway, which is deregulated in numerous cancers including breast, prostate, and lung cancers, these therapies aim to obstruct critical pathways involved in cancer cell growth and survival89. The integration of mTOR inhibitors with other anticancer agents has been shown to mitigate therapy resistance, providing a formidable defense against the evolution of resilient cancer cells9.
Emerging Cancer Treatments Involving mTOR Inhibition
The advent of emerging cancer treatments involving mTOR inhibition highlights a promising frontier in oncology. Clinical trials have underscored the efficacy of next-generation mTOR inhibitors that exhibit potent anti-tumor activity by extensively disrupting cancer cell metabolism and signaling pathways910. Furthermore, these agents are part of investigational strategies aimed at overcoming resistance to traditional therapies, indicating significant advancements in extending progression-free survival for patients with complex cancers810.
As ongoing studies and trials continue to illuminate the benefits of mTOR inhibition therapies, the landscape of oncology is reshaped, promising a future where cancer treatment is not only targeted but also more adaptive to the challenges posed by drug resistance and tumor diversity.8910
Advancements in Cancer Treatment: Resistance Mechanisms to mTOR Inhibitors
Recent developments in cancer treatment advancements have significantly focused on the intricacies of resistance mechanisms that hinder the efficacy of mTOR inhibitors. Research indicates that genetic variations and alterations in signaling pathways are pivotal in developing resistance, necessitating novel strategies for intervention. Explore detailed genetic insights here.
The rising incidence of cancer and its related mortalities worldwide presses the need for continuous innovation in treatment modalities11. Studies have demonstrated that endocrine therapy, while effective initially, often faces resistance, leading to treatment failure in breast cancer cases11. Similarly, mechanisms involving multidrug resistance present substantial challenges in cancer chemotherapy, driven by complex genetic and molecular interactions11.
Understanding these resistance networks, facilitated by network biology and artificial intelligence, has provided a clearer picture of the multidrug resistance phenotype in various cancers11. This insight is crucial for developing next-generation mTOR inhibitors that can potentially overcome these hurdles and improve therapeutic outcomes. Clinical trials are underway to explore these opportunities, aiming to harness these advanced inhibitors in real-world scenarios effectively.
- Genetic mutations play a significant role in resistance to mTOR inhibitors.
- Activation of compensatory signaling pathways can diminish the effectiveness of current treatments.
- Research is increasingly focusing on feedback loops that could potentially be exploited to reverse resistance mechanisms.
The pathway forward in cancer treatment not only involves understanding these resistance mechanisms but also adapting our approaches to remain effective against evolving oncological challenges. As we gain more insights, the development of more robust mtor inhibitors promises a new era of efficacy in cancer therapy.
| Resistance Mechanism | Impact on Treatment | Potential Solution |
|---|---|---|
| Genetic mutations | Decreases drug efficacy | Targeted genetic therapies |
| Altered signaling pathways | Leads to adaptive resistance | Combination drug therapies |
| Activation of feedback loops | Compensates for drug action | Novel inhibitory agents |
Your understanding of these complexities and continuous research efforts are essential in the fight against cancer, pushing the boundaries of what is possible in oncological care and mTOR inhibitors.
Cancer Treatment Milestones: The Evolution of mTOR Targeting Drugs
The journey of mTOR targeting drugs in oncology highlights a critical stage in the ongoing battle against cancer. These drugs, particularly PI3K/AKT/mTOR inhibitors, have carved a niche in breast cancer clinical trials, offering new perspectives and possibilities in treatment approaches.
PI3K/AKT/mTOR Inhibitors in Breast Cancer Clinical Trials
Recent advancements in mTOR targeting drugs have profoundly impacted the landscape of breast cancer treatment. The inclusion of PI3K/AKT/mTOR inhibitors in clinical trials has provided promising outcomes in the fight against this pervasive disease. These inhibitors target the critical pathways involved in cell growth and survival, which are often deregulated in cancer cells. The integration of these inhibitors into treatment protocols aims to enhance therapeutic efficacy and curb the prevalent issue of drug resistance, potentially leading to more sustainable patient outcomes.
Case Studies: Successes and Setbacks in mTOR Inhibition Therapy
Investigations into mTOR inhibition have yielded a wealth of data, encapsulated in various case studies that demonstrate both successes and setbacks. These studies reveal a spectrum of responses, where some patients exhibit significant improvement while others may encounter resistance to the therapy. The nuanced outcomes underscore the necessity for ongoing research and adaptation of therapeutic strategies, particularly in harnessing the potential of mTOR targeting drugs for optimized cancer care.
The statistical insights from extensive research have shown that the mTOR protein kinase not only regulates cell growth, survival, and metabolism but also plays a pivotal role in the immunological aspects of cancer cells, critical insights that have helped refine mTOR inhibition strategies in clinical settings8. Moreover, the discovery that mTOR signaling pathway is frequently deregulated in various human cancers emphasizes the urgent need for targeted therapeutic approaches in oncology8.
Emerging research highlights the role of the PI3K/AKT pathway as a principal target in cancer treatment strategies, creating a foundational understanding that guides the development of inhibitors aiming at this pathway12. Additionally, the exploration of mTOR’s cross-talk within cancer cells presents opportunities for combination therapies, which could potentially overcome the intrinsic resistance seen with traditional treatments12.
As we continue to explore the efficacy and safety of mTOR inhibitors, the lessons learned from past trials and current studies will be instrumental in guiding future clinical practices and therapeutic development. The collective efforts in this field are crucial for realizing the full potential of mTOR targeting drugs in cancer treatment, ensuring that each milestone reached can lead to better patient outcomes in the ongoing fight against cancer.
mTOR Pathway Inhibitors: FDA-Approved Drugs and Their Effects
The treatment landscape for various cancers has been significantly revolutionized by FDA-approved mTOR pathway inhibitors such as everolimus and alpelisib. These drugs are pivotal in the fight against cancer due to their targeted approach in the mTOR signaling pathway.
Evaluating the Efficacy and Safety of mTOR Inhibitors
When considering drug efficacy, mTOR pathway inhibitors have demonstrated substantial success in slowing disease progression in cancers like breast and renal cell carcinoma. However, the safety evaluations of these drugs are equally vital to ensure they provide a favorable benefit-risk balance for patients. Adverse effects, while manageable, require careful monitoring by healthcare professionals.
Understanding the Pharmacodynamics of mTOR Pathway Drugs
The pharmacodynamics of mTOR inhibitors are complex but crucial for predicting how different patients might respond to therapy. Factors such as the presence of PIK3CA somatic mutations in breast cancer play a significant role in drug responsiveness and are essential markers for personalizing treatment approaches.2 Additionally, understanding how these drugs interact with the body can guide dosing adjustments and manage patient expectations regarding possible side effects.
Global insights into the prevalence of PI3K-AKT-mTOR pathway mutations, like those found in head and neck cancer, inform drug choice and combinations, potentially enhancing treatment efficacy and safety for a broad patient demographic.2
Note: For more detailed studies on the mTOR pathway, refer to this comprehensive analysis.
Current Trends in mTOR Pathway Research and Related Clinical Trials
The landscape of molecular oncology is continuously evolving with significant advancements in mTOR pathway research, highlighting a surge in clinical trials that focus on combating cancer more effectively. As you delve into the latest research, it’s evident that the hyperactivation of the PI3K/AKT/mTOR pathway plays a central role in cancer progression and drug resistance, particularly in breast cancer, which remains the leading cause of cancer death among women globally1.
One of the crucial elements in this pathway is the PIK3CA gene, known to be one of the most frequently mutated genes in breast cancers1. This genetic alteration paves the way for the development of targeted therapies such as PIK3 and AKT inhibitors, which are currently under intense investigation in various clinical trials. These trials aim to evaluate the inhibitors’ efficacy across different patient demographics and stages of treatment1.
Emerging from recent trials, specific inhibitors such as alpelisib, taselisib, and pictilisib have demonstrated improved tolerability and significantly enhanced anticancer efficacy, thereby promising a new frontier in cancer treatment strategies1. Furthermore, everolimus, the first mTOR inhibitor approved for HR+/HER2- breast cancer, illustrates the potential of targeting specific pathways to improve treatment outcomes1kk>.
In the ongoing pursuit of extending life and improving the quality of care, it is noteworthy that alpelisib has notably improved median progression-free survival in patients with PIK3CA-mutated advanced breast cancer1. This data not only underscores the importance of molecular profiling in cancer treatment but also reinforces the need for ongoing clinical trials to refine and enhance the efficacy of existing and new therapeutic modalities.
To stay at the forefront of current trends in cancer research, it is crucial to continuously monitor and participate in the advancements within mTOR pathway research. These efforts are not just transforming the landscape of molecular oncology, but are also crucial in shaping the future of personalized medicine, where treatments are tailored to the genetic makeup of individual tumors, offering hope for better management of this complex disease.
Unlocking the Potential of Molecular Oncology in Managing Resistance
The field of molecular oncology is pivotal in the fight against cancer, particularly in crafting resistance management strategies that adapt to the complexities of cancer treatments. With extensive research and case studies, it becomes evident how genetic variations and molecular dynamics play critical roles in resisting conventional therapies, thereby influencing the development of robust therapeutic strategies.
Case Studies of Resistance Mechanisms in Cancer Treatments
Insight into resistance mechanisms is crucial for understanding the challenges posed by targeted therapies. For instance, the global statistics of cancer incidences and mortalities highlight the need for accelerated efforts in resistance management13. Studies have shown that targeted therapy combinations in early-phase trials often face hurdles due to the adaptive nature of cancer cells, which evolve to bypass the drug’s action13.
Moreover, insights into the apoptotic genes targeted by silibinin-loaded polymeric nanoparticles, for example, showcase promising avenues for overcoming drug resistance in breast cancer treatments through cell death induction mechanisms13. Such research efforts highlight the nuanced approaches needed to dismantle cancer cells’ resistance capabilities effectively.
Future Directions in Therapeutic Strategies and Biomarker Research
Advancements in biomarker research are setting the stage for revolutionary changes in cancer therapy. The development and characterization of Rutin loaded PCL-PEG nanoparticles against ovarian cancer cells offer a glimpse into the potential of nano-formulated agents to improve clinical outcomes by targeting disease on a molecular level13. Furthermore, the ongoing assessment and integration of biomarkers, such as microRNA-132 and Cyclin D1 in therapeutic protocols, pave the way for personalized and more effective treatment modalities13.
Molecular oncology
continues to push the boundaries of modern medicine by integrating
biomarker research
into the development of
therapeutic strategies
, thus ensuring that future treatments are not only effective but also adaptable to the complex nature of cancer evolution and resistance dynamics. These endeavors are essential for the ultimate goal of achieving personalized medicine, which promises to tailor treatments to individual genetic profiles, optimizing outcomes in the battle against cancer.
How editverse.com can help you to do research?
When delving into intricate medical subjects like the mTOR pathway or examining resistant mechanisms in cancer therapy, finding credible and comprehensive resources is crucial. Here’s where editverse.com becomes an indispensable tool for your research assistance. With access to thousands of academic papers and articles, this platform equips you with necessary cancer therapy insights, enhancing your depth of understanding and range of knowledge in molecular oncology.
Additionally, the extensive database at editverse.com is replete with case studies and clinical trial reports, providing a robust foundation for research support. If you’re exploring the latest drugs approved for targeting oncological pathways or understanding their efficacy and resistance patterns, such as PI3K inhibitors alpelisib, idelalisib, and copanlisib, which have been noted for their role in pharmaceutical therapy1, editverse.com’s wealth of information is invaluable.
Engaging with editverse.com further simplifies the process of gathering information on pivotal studies, such as those focusing on metabolic inhibitors like 2DG and DCA in cancer cells showing resistance to traditional treatments10. This platform not only provides data but also contexts that are critical for shaping effective cancer therapy strategies and understanding molecular interactions.
Whether your focus is on PI3K/AKT/mTOR inhibitors and their classifications or mTOR’s role in drug resistance mechanisms, editverse.com’s comprehensive resources empower you with up-to-date information that is essential for both academia and clinical applications. By ensuring that these resources are readily accessible, editverse.com significantly augments your ability to conduct thorough and informed research.
| Resource Type | Description | Utility in Cancer Research |
|---|---|---|
| Academic Articles | Detailed discussions on recent advances in mTOR inhibitors | Insights into drug mechanisms and resistance profiles |
| Clinical Trial Data | Updates on the latest trials involving PI3K/AKT/mTOR pathways | Progression-free survival rates and drug efficacies1 |
| Case Studies | Real-world outcomes of therapies on resistant cancer types | Strategies for managing therapy-resistant patients |
In summary, leveraging the power of editverse.com for your research into molecular oncology and cancer therapy not only simplifies your access to comprehensive resources but also enhances the quality and effectiveness of the insights you gain.”]))
Conclusion
In the ongoing battle against cancer, the mTOR pathway has emerged as a critical frontier in both understanding and combating drug resistance. The multifaceted nature of this pathway impacts key aspects of oncogenesis, including tumorigenesis, cell growth, and metabolism. The evidence is mounting that the mTOR kinase, operating through mTORC1 and mTORC2, is not only implicated in the resistance to therapeutics targeted against oncogenes but also serves as a central mediator in the translation of proteins that govern cell cycle and survival1415. These insights are guiding ongoing clinical trials, offering new avenues for overcoming drug resistance in treatment-resistant forms of lung cancer, breast cancer, and melanoma14.
Despite significant advancement in cancer therapy, nearly half of all patients experience therapy failure or tumor relapse14. In response to this challenge, the shift towards targeted molecular mechanisms, specifically second-generation mTOR inhibitors, holds promise14. These advanced inhibitors aim to avert the severe toxicities associated with their predecessors, though the path is paved with complexity concerning efficacy and patient-specific responses. Current research is also unveiling how cancer cells’ metabolic alterations, such as the upregulation of glycolysis, unveil vulnerabilities that can be exploited by metabolic inhibitors like 2DG and DCA, leading to apoptosis in chemotherapy-resistant cells14.
As our oncology insights deepen, clinicians and researchers are poised to harness cancer therapy advancements to improve patient care. Tackling the mTOR pathway’s integral role in cancer biology, new therapies are actively being developed to exploit druggable vulnerabilities and inhibit cancer cell proliferation—despite the myriad of resistance mechanisms that have previously hindered success. The mTOR pathway conclusion is clear: by continuing to refine and integrate targeted treatments, based on molecular pathology, we are making significant strides in the quest to overcome drug resistance and enhance the efficacy of cancer therapeutics15.
FAQ
What is the mTOR pathway and why is it important in oncology?
The mTOR (mechanistic target of rapamycin) pathway is a critical cell signaling pathway that regulates cell growth, proliferation, and survival. In oncology, it is significant because it is often dysregulated in cancer cells, leading to tumor progression and drug resistance. Clinicians and researchers focus on this pathway to develop targeted therapies and improve cancer treatment outcomes.
How does the mTOR pathway contribute to drug resistance in cancer treatments?
The mTOR pathway can contribute to drug a resistance through several mechanisms, including overexpression of resistance proteins, activation of alternative signaling pathways, and alterations in cell cycle checkpoints. Hyperactivation of the PI3K/AKT/mTOR pathway, for instance, is known to hinder the effectiveness of various cancer treatments, making the targeting of this pathway a priority in overcoming resistance.
What are some of the FDA-approved mTOR pathway inhibitors used in cancer therapy?
FDA-approved mTOR inhibitors include everolimus and alpelisib. These drugs are used in the treatment of certain cancers, such as advanced hormone receptor-positive, HER2-negative breast cancer. They work by targeting the mTOR pathway to inhibit cancer cell growth and proliferation.
How are clinical trials advancing our understanding of the mTOR pathway in oncology?
Clinical trials play a crucial role in evaluating the safety and efficacy of drugs targeting the mTOR pathway. They contribute to our understanding of how mTOR inhibitors can be integrated with current therapies, their potential in overcoming drug resistance, and the overall improvement of patient outcomes in cancer treatment.
What are the current trends in mTOR pathway research?
Current research trends in the mTOR pathway include the development of more potent and selective inhibitors, exploring the pathway’s relationship with other biological processes, and the use of combination therapies to combat drug resistance. Precision medicine approaches that involve identifying biomarkers for targeted interventions are also at the forefront.
Can targeting the mTOR pathway lead to personalized cancer treatment?
Yes, targeting the mTOR pathway is central to the concept of precision medicine in oncology. Identifying specific biomarkers within the mTOR pathway can aid in tailoring treatments to the individual molecular profiles of a patient’s tumor, which can enhance treatment efficacy and potentially reduce the likelihood of resistance.
What therapeutic strategies are being developed to overcome resistance mechanisms associated with the mTOR pathway?
Strategies to overcome resistance include developing combination therapies that target multiple points within the mTOR pathway and related signaling networks. By disrupting compensatory pathways and downstream effectors, these combined therapeutic approaches aim to maintain efficacy against resistant cancer cells.
How can editverse.com assist with research on the mTOR pathway in oncology?
Editverse.com offers comprehensive resources that provide detailed insights into various facets of the mTOR pathway in oncology, including drug resistance, clinical trials, and emerging treatments. Its platform can be a valuable tool for anyone looking to deepen their understanding of molecular oncology and stay updated on the latest research developments.
Source Links
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8005514/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8987494/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7063815/
- https://www.nature.com/articles/s41392-024-01760-0
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8699328/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7272286/
- https://cellandbioscience.biomedcentral.com/articles/10.1186/s13578-020-00396-1
- https://jhoonline.biomedcentral.com/articles/10.1186/s13045-019-0754-1
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4128044/
- https://www.nature.com/articles/s41467-020-18504-7
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10203373/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6387042/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11020265/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7499183/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2519122/

