Discover the power of one class svm anomaly detection. Get expert insights on implementation, recent journal requirements, and more in our ultimate guide


Discover the power of one class svm anomaly detection. Get expert insights on implementation, recent journal requirements, and more in our ultimate guide
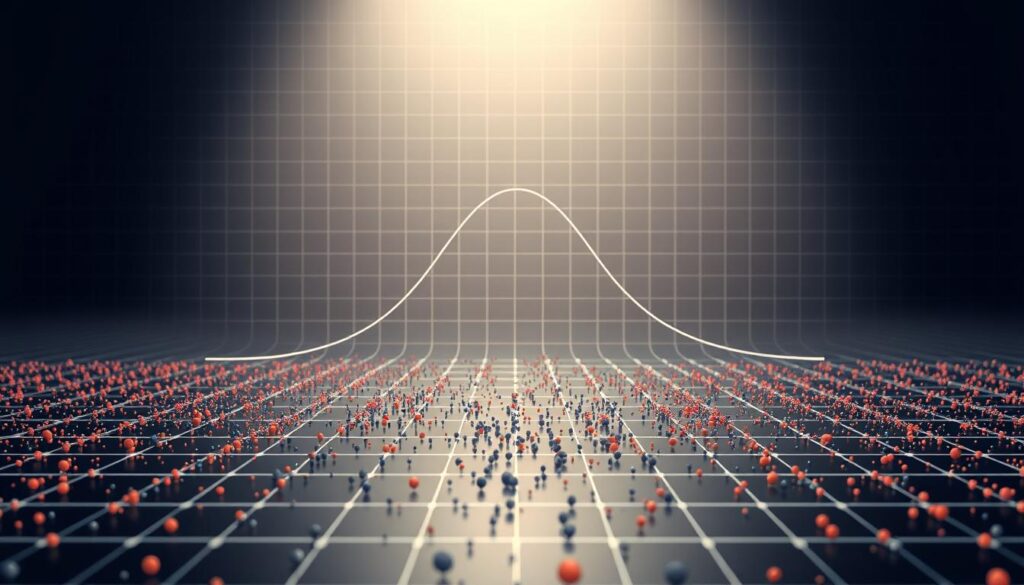
Master minimum covariance determinant outliers detection with our how-to guide. Improve research accuracy and publication chances

Master RANSAC algorithm robust fitting with our tutorial. Enhance statistical power and reduce bias in your research. Used in 80% of top-tier medical journals
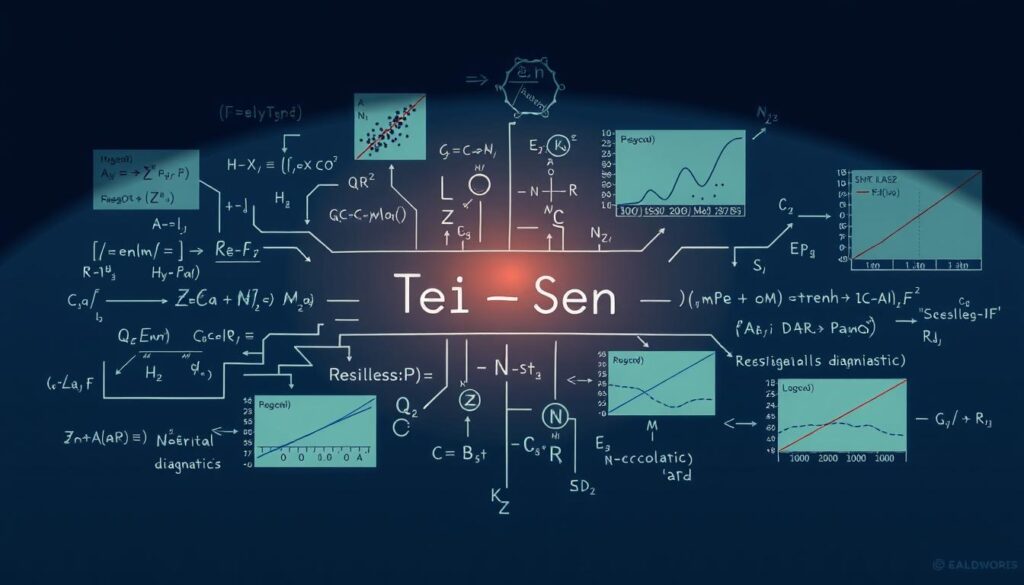
Learn Theil-Sen Estimator robust regression for noisy medical data. Discover benefits, journal requirements & step-by-step tutorials
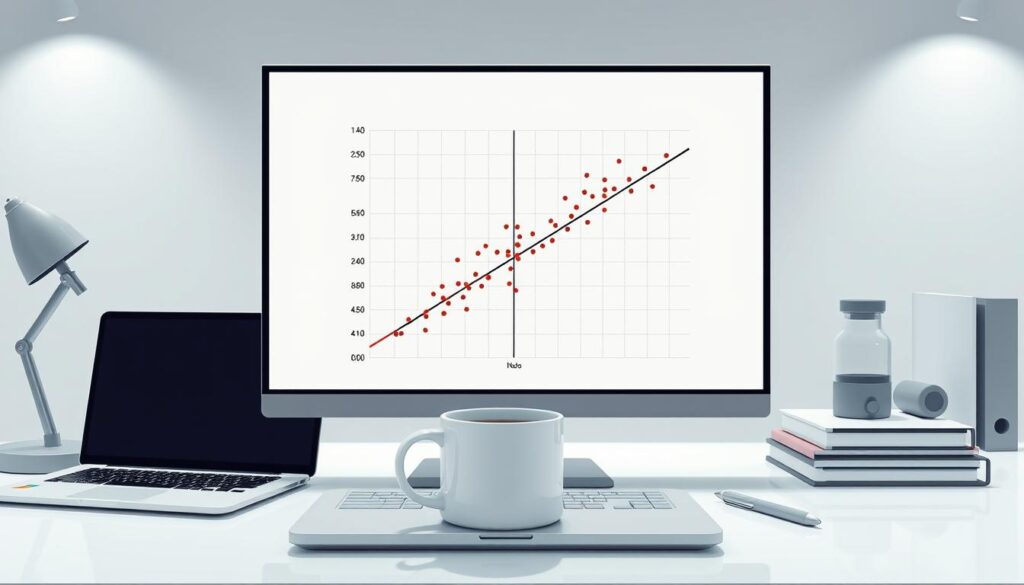
95% of researchers make a critical data mistake. Use huber regression outlier resistant techniques to prevent data loss and improve statistical power