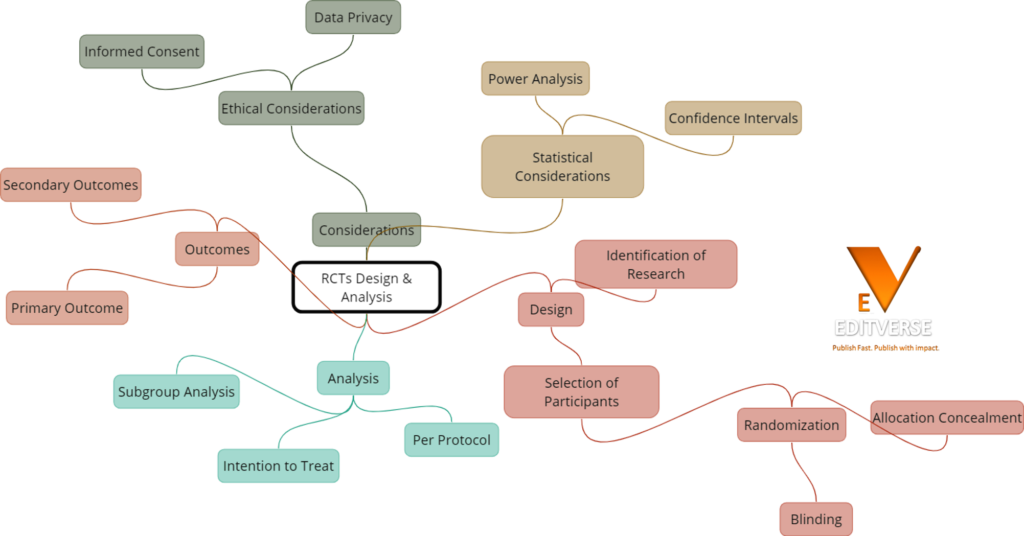
Did you know that randomized controlled trials (RCTs) are considered the gold standard in clinical research methodology? These rigorous experimental studies have revolutionized the field of evidence-based medicine, providing reliable and unbiased evidence to guide medical decision-making. In this article, we will delve into the crucial aspects of RCT design and analysis, uncovering the significance of randomization, different study designs, and strategies to strengthen internal validity. Join us as we explore the fascinating world of RCTs and their impact on advancing healthcare.
Key Takeaways:
- Randomized controlled trials (RCTs) are a fundamental research methodology in clinical research.
- RCTs employ randomization to minimize bias and increase the internal validity of study findings.
- Different RCT designs, such as parallel, crossover, and factorial designs, allow for the evaluation of intervention effects.
- Strengthening internal validity in RCTs involves controlling confounding variables and designing well-defined research questions.
- Efficacy and effectiveness are distinct concepts in evaluating intervention impact, with explanatory and pragmatic trials catering to different research objectives.
Understanding the Foundations of RCT Methodology
Randomized controlled trials (RCTs) are essential in clinical trials research, providing robust evidence for evaluating the efficacy and effectiveness of interventions. To fully comprehend the methodology behind RCTs, it is crucial to understand the significance of randomization, the prospective nature of these trials, and the different designs employed.
To make your manuscript great, you can take advantage of www.editverse.com
The Significance of Randomization in Clinical Trials
Randomization is a vital process in RCT methodology that aims to minimize bias and increase the internal validity of a study. By randomly assigning participants to different intervention groups, the randomization process ensures that each individual has an equal chance of being allocated, reducing the risk of confounding variables affecting the study outcomes. This process helps create comparable groups and strengthens the validity of the findings.
Prospective Nature of Randomized Clinical Trials
Randomized clinical trials are prospective in nature, meaning that data is collected over time. This design allows for a systematic and standardized approach to exposure and outcome measurement. By following participants over a defined period, RCTs provide valuable insights into the short-term and long-term effects of interventions, contributing to evidence-based medicine and clinical decision-making.
Parallel, Crossover, and Factorial Designs in RCTs
RCTs can utilize different designs to evaluate intervention effects and control groups. Three common designs include parallel group design, crossover design, and factorial design.
Parallel group design involves randomly assigning participants to different intervention groups, where each group receives a specific treatment or control. This design allows for straightforward comparisons between the groups.
Crossover design involves participants receiving different interventions in a specific order, with a washout period in between. This design helps evaluate the effects of interventions within the same individuals and reduces between-subject variability.
Factorial design incorporates multiple interventions and control groups, allowing researchers to evaluate the main effects of each intervention, as well as potential interactions between them. This design provides valuable insights into the combined effects of multiple treatments.
Each design has its advantages and limitations, and the choice of design depends on the research question, the complexity of the interventions, and the available resources.
| Design | Description |
|---|---|
| Parallel Group Design | Randomly assigns participants to different intervention groups, enabling straightforward comparisons. |
| Crossover Design | Participants receive different interventions in a specific order, with a washout period, reducing variability. |
| Factorial Design | Incorporates multiple interventions and control groups to evaluate main effects and potential interactions. |
Strengthening Internal Validity Through RCT Design
Internal validity plays a crucial role in the design and implementation of Randomized Controlled Trials (RCTs). It refers to the extent to which observed differences between intervention and control groups can be attributed to the intervention itself. By strengthening internal validity, RCTs ensure that the results accurately reflect the effects of the interventions being studied.
One of the primary ways RCTs enhance internal validity is through the process of randomization. Randomization involves the random assignment of participants to different intervention or control groups. This random assignment helps to minimize the impact of both known and unknown confounding variables, reducing bias and increasing the confidence in the causal relationships being examined.
Designing an RCT with a well-defined research question is essential to maintain internal validity. A clearly defined research question sets the groundwork for the study and ensures that the goals and objectives of the research are clearly defined. This clarity helps to guide the design, implementation, and interpretation of the study, enhancing its internal validity.
Standardized protocols and clear parameters for data collection and analysis also contribute to strengthening internal validity. By using standardized protocols, researchers can ensure consistency in the implementation of interventions and the measurement of outcomes. Clear parameters for data collection and analysis help to minimize bias and enhance the validity of the study results.
Overall, the design of an RCT should prioritize internal validity to ensure that the observed differences between intervention and control groups can be confidently attributed to the intervention itself. By employing randomization, controlling for confounding variables, and utilizing robust research design, RCTs can provide high-quality evidence for decision-making in clinical practice.
The Distinction Between Efficacy and Effectiveness in RCTs
In the field of clinical research, it is important to understand the difference between efficacy and effectiveness when assessing the impact of interventions. Efficacy refers to the performance of an intervention under ideal and controlled circumstances, typically evaluated through explanatory trials. These trials employ strict inclusion criteria and standardized protocols to minimize confounding factors and assess the intervention’s effectiveness. On the other hand, effectiveness measures the intervention’s performance under real-world conditions, where pragmatic trials are conducted. These trials aim to assess the intervention’s impact in diverse patient populations and real-world settings.
Defining Efficacy in Clinical Research Methods
Efficacy, as defined in clinical research methods, emphasizes the intervention’s performance in controlled settings. Explanatory trials are designed with strict inclusion criteria and standardized protocols, minimizing the influence of confounding factors. The primary goal of efficacy trials is to determine if an intervention is capable of producing the desired therapeutic effect when delivered optimally, without external influences.
Measuring Effectiveness in Real-World Conditions
Effectiveness, in contrast, focuses on evaluating the intervention’s performance in real-world conditions and diverse patient populations. Pragmatic trials aim to assess the intervention’s impact in these real-world settings, where patient characteristics and external factors may influence the intervention’s outcomes. By evaluating effectiveness, researchers can gather insights into how the intervention performs in everyday clinical practice and its generalizability to a broader population.
Designing Explanatory and Pragmatic Trials
Designing both explanatory and pragmatic trials requires careful consideration of various factors. Explanatory trials are designed to provide high internal validity by minimizing confounding factors and ensuring the intervention’s efficacy. On the other hand, pragmatic trials are designed to assess the intervention’s effectiveness in real-world conditions. The choice between these trial designs depends on the research question, the desired level of generalizability, and the specific population being studied.
Strategies for Patient Allocation and the Randomization Process
Patient allocation in randomized controlled trials (RCTs) is a critical step in ensuring the validity and reliability of study outcomes. Randomization techniques are employed to assign patients to different intervention groups or control groups, minimizing bias and enhancing the internal validity of the study.
Simple vs. Complex Randomization Techniques
In the randomization process, simple techniques are commonly used, such as coin flipping or computer-generated random numbers. These techniques provide an equal chance for each patient to be assigned to a group. However, simple randomization may not always be appropriate, especially in studies with specific objectives or complex designs.
Complex randomization techniques, such as blocking or stratification, can further enhance the randomization process. Blocking involves segregating participants into small groups based on specific characteristics, like age or severity of the condition, before randomization. Stratification, on the other hand, ensures a balanced distribution of patients across different groups based on specific variables, such as age or gender, to reduce potential confounding variables.
Preventing Bias with Adequate Randomization
Adequate randomization is crucial in preventing selection bias, ensuring that each participant has an equal chance of being assigned to different groups. By employing randomization techniques, potential confounding factors are evenly distributed among the groups, minimizing the influence of these factors on the study outcomes. This reduces bias in the results and enhances the internal validity of the study.
Implications of Randomization on Study Outcomes
The randomization process directly impacts the study outcomes in randomized controlled trials. By ensuring a balanced distribution of participants across intervention and control groups, randomization minimizes the influence of confounding variables on the study outcomes. This allows for a more accurate assessment of the effects of the intervention and improves the overall validity and generalizability of the study results.
By implementing appropriate strategies for patient allocation and the randomization process, researchers can strengthen the design of their RCTs and enhance the reliability and validity of their study outcomes.
Critical Elements of Experimental Study Design
When designing randomized controlled trials (RCTs), it is crucial to consider the critical elements of experimental study design to ensure the validity and reliability of study findings. These elements play a vital role in producing robust evidence for clinical practice and decision-making. The key elements include:
- Control Group: Including a control group is essential in RCTs as it provides a baseline for comparison against the intervention group. The control group receives either no treatment, a placebo, or an alternative treatment. By comparing the outcomes between the intervention and control groups, researchers can evaluate the effectiveness of the intervention.
- Intervention Group: The intervention group receives the treatment or intervention being evaluated in the RCT. Comparing the outcomes of the intervention group with those of the control group helps assess the efficacy and safety of the intervention.
- Blinding: Blinding refers to the practice of withholding information from participants, researchers, or both, to minimize bias in the study. In double-blind trials, neither the participants nor the researchers know who is receiving the intervention or control. Blinding helps reduce the influence of expectations and preconceived notions on study outcomes, enhancing the validity of the results.
- Allocation Concealment: Allocation concealment ensures that the process of assigning participants to intervention or control groups remains concealed from the researchers until the moment of randomization. This practice prevents selection bias and maintains the integrity of the randomization process.
By carefully considering these critical elements during the design phase of an RCT, researchers can enhance the internal validity, reliability, and generalizability of their study findings. These elements contribute to producing high-quality evidence that informs clinical decision-making and improves patient outcomes.
Table: Critical Elements of Experimental Study Design
| Element | Description |
|---|---|
| Control Group | Includes a baseline comparison group receiving no treatment, a placebo, or an alternative treatment. |
| Intervention Group | Receives the treatment or intervention being evaluated in the RCT. |
| Blinding | Withholds information from participants and researchers to minimize bias. |
| Allocation Concealment | Ensures that the process of assigning participants to groups remains concealed until randomization, preventing selection bias. |
Building a Robust Study Team for Effective RCT Execution
The successful execution of a Randomized Controlled Trial (RCT) requires the collaboration of a well-coordinated and interdisciplinary study team. Each member plays a vital role in ensuring the clinical relevance and methodological rigor of the trial. Let’s explore the key roles and responsibilities within an RCT study team.
Role of Clinicians and Researchers in RCTs
Clinicians are essential in RCTs for their expertise in patient care and clinical decision-making. They contribute valuable insights during the trial’s design phase, ensuring that the intervention aligns with clinical practice and addresses relevant research questions. Clinicians also play a crucial role in recruiting participants, implementing the intervention, and collecting data related to clinical outcomes.
Researchers, on the other hand, contribute their scientific knowledge and research expertise to the RCT. They collaborate with clinicians to design the study protocol, define the research question, and establish outcome measures. Researchers are responsible for ensuring methodological rigor, data analysis, and interpretation of the study results. Their scientific expertise is instrumental in producing high-quality evidence for clinical decision-making.
Importance of Involving Statisticians Early in Clinical Trials Research
Statisticians are key players in the successful execution of RCTs. Their involvement in the early stages of study planning helps in the selection of appropriate statistical methods and sample size calculations. Statisticians provide guidance on randomization techniques, data management, and statistical analysis plans. Their expertise ensures that the study design and statistical analysis align with the research objectives, maximizing the validity and reliability of the study findings.
Managing Multi-Center Trials and Coordinating Research Efforts
Multi-center trials involve collaboration among multiple research sites, each with its own clinicians, researchers, and support staff. Coordinating research efforts across these sites is crucial to maintaining consistency in study protocols, data collection procedures, and participant recruitment. Effective communication and coordination among the study team members and research sites are necessary to ensure unified study execution and minimize variations in data collection and intervention implementation.
Research coordination involves overseeing the overall management of the trial, including study timeline adherence, data monitoring, and ensuring compliance with ethical standards and regulatory requirements. Research coordinators play a key role in streamlining communication among team members, managing study logistics, and maintaining documentation. Their role is crucial in ensuring the smooth functioning of the RCT and optimizing the data collection process.
| Role | Responsibilities |
|---|---|
| Clinicians | Provide clinical expertise, recruit participants, implement interventions, and collect clinical outcome data |
| Researchers | Contribute scientific knowledge, design the study protocol, analyze data, and interpret study results |
| Statisticians | Provide statistical expertise, assist in study design, sample size calculation, randomization techniques, and data analysis |
| Research Coordinators | Oversee trial management, communicate with team members and research sites, manage logistics, and ensure compliance |
By establishing a well-structured and collaborative study team, an RCT can be executed effectively, ensuring the collection of high-quality data and the generation of reliable evidence to inform clinical practice. The involvement of clinicians, researchers, statisticians, and research coordinators at different stages of the trial is vital for its success.
Setting Inclusion and Exclusion Criteria for Meaningful Control Group Comparison
Inclusion and exclusion criteria play a crucial role in Randomized Controlled Trials (RCTs) by defining the study population and establishing meaningful comparisons between the intervention and control groups.
Generalizability vs. Minimization of Bias
When setting inclusion and exclusion criteria in RCTs, a balance must be struck between generalizability and minimization of bias. Generalizability ensures that the study results can be applied to a broader population, while minimizing bias strengthens the internal validity of the study by reducing confounding factors.
Factors Influencing Eligibility Criteria in Clinical Trials
Several factors influence eligibility criteria in clinical trials. Patient characteristics, disease severity, comorbidities, and other relevant factors must be considered to ensure that the study population accurately represents the target population.
Optimizing Criteria for Targeted Intervention Outcomes
Optimizing the inclusion and exclusion criteria helps target the intervention outcomes of interest. By carefully defining the criteria, researchers can ensure that the study population is more likely to respond to the intervention, increasing the external validity of the study findings.
Ensuring High-Quality Data Collection and Analysis in RCTs
High-quality data collection and analysis are essential in producing reliable and valid results in Randomized Controlled Trials (RCTs). To achieve this, it is crucial to implement proper processes and protocols for data collection that ensure data integrity and accuracy. By adhering to rigorous data management and quality assurance measures, researchers can maintain the integrity of the collected data throughout the study.
When it comes to data analysis in RCTs, the selection of appropriate statistical techniques is vital. These techniques should be tailored to the study’s pre-defined endpoints and focus on handling the data effectively to avoid biases. By employing robust statistical analysis methods, researchers can generate meaningful interpretations of the study findings.
Research studies have shown that proper data collection and analysis methods significantly impact the reliability and validity of study results. In a study published by the NCBI, it was found that meticulous attention to data collection protocols reduced the likelihood of missing data and improved the accuracy of analysis results [source].
| Data Collection | Data Analysis | Quality Assurance | Data Management | Statistical Analysis |
|---|---|---|---|---|
| Develop standardized protocols for data collection | Select appropriate statistical techniques | Implement quality control procedures | Ensure data integrity and accuracy | Apply statistical methods tailored to study endpoints |
| Train research staff on proper data collection methods | Handle and clean data to remove errors and outliers | Conduct regular data audits and checks | Securely store and organize data | Account for potential biases and confounding factors |
| Use standardized data collection forms | Perform sensitivity analyses to assess robustness | Ensure compliance with ethical and regulatory guidelines | Back up data regularly to prevent loss | Consider the power and sample size requirements |
Implementing high standards for data collection and analysis enhances the credibility and reliability of RCT findings, strengthening the evidence base for clinical practice and decision-making. By following rigorous protocols and employing appropriate statistical techniques, researchers can confidently draw conclusions from their studies, providing valuable insights into effective interventions.
Establishing Clear Parameters for Statistical Analysis in RCTs
In order to ensure the validity and reliability of study results in Randomized Controlled Trials (RCTs), it is crucial to establish clear parameters for statistical analysis. By defining these parameters, researchers can effectively analyze the data collected and derive meaningful insights from the study. This section will explore the importance of predefined endpoints, strategies for handling data and avoiding bias, and the interpretation of statistical significance and clinical relevance in RCTs.
Importance of Predefined Endpoints
Predefined endpoints play a vital role in focusing the data analysis of an RCT. These endpoints are specific outcomes of interest that researchers plan to measure and evaluate during the study. By clearly defining these endpoints before the trial begins, researchers can ensure that the analysis is aligned with the objectives of the study and targeted towards assessing the desired outcomes. This helps in maintaining the focus of the study and maximizing the relevance of the statistical analysis.
Strategies for Handling Data and Avoiding Bias
Appropriate strategies for handling data are essential in RCTs to avoid bias and ensure the validity of the study results. Researchers should address any missing data through appropriate techniques, such as imputation or sensitivity analysis, to ensure that the analysis is not compromised. Additionally, using appropriate statistical techniques, such as intention-to-treat analysis, can help prevent bias by including all randomized participants in the analysis, regardless of compliance with the assigned intervention. Other strategies, such as blinding and randomization, can further minimize bias and enhance the quality of the statistical analysis.
Interpreting Statistical Significance and Clinical Relevance
Interpreting statistical significance and clinical relevance in the context of RCTs requires a comprehensive understanding of statistical methods and their implications for clinical practice. Statistical significance refers to the probability that the observed differences between intervention groups are not due to chance. However, statistical significance does not automatically imply clinical relevance. It is important to assess the magnitude and clinical significance of the observed effects. Researchers need to consider both statistical significance and clinical relevance when drawing conclusions from the study findings, ensuring that the results have practical implications and can guide decision-making in clinical practice.
| Key Considerations for Statistical Analysis in RCTs |
|---|
| Predefine endpoints to focus data analysis on specific outcomes of interest |
| Address missing data using appropriate techniques to avoid bias |
| Utilize statistical techniques like intention-to-treat analysis and blinding to minimize bias |
| Interpret statistical significance in the context of clinical relevance |
Placebo-Controlled Trials Versus Active Comparators
When conducting randomized controlled trials (RCTs), researchers often encounter the decision of using placebo-controlled trials or trials with active comparators. Both approaches have their advantages and limitations and play significant roles in ensuring trial validity and ethical considerations.
Advantages and Limitations of Placebo Use
Placebos are inert substances or treatments that have no specific therapeutic effect. The use of placebos in RCTs allows researchers to assess if the intervention under study has a specific effect beyond patient expectations and natural healing processes. The advantages of placebo-controlled trials include:
- Eliminating bias: Placebo controls help to eliminate bias by providing a baseline for comparison to evaluate the true effect of the intervention.
- Evaluating true treatment effect: Placebos help determine whether the observed treatment effects are due to the intervention itself or other factors.
- Minimizing confounding variables: Placebo controls help minimize confounding variables that can influence the study outcomes, providing a more accurate assessment of the intervention’s efficacy.
However, placebo-controlled trials also have limitations. The use of placebos may raise ethical concerns as participants assigned to the placebo group may not receive potentially beneficial treatments. Ethical considerations should be carefully evaluated to protect the rights and well-being of trial participants.
Choosing Appropriate Comparators to Enhance Trial Validity
In contrast to placebo-controlled trials, trials with active comparators involve comparing the intervention under study with existing standard treatments or alternative interventions. Active comparators serve to assess the superiority or non-inferiority of the intervention. The choice of appropriate comparators is crucial to enhance trial validity and generate meaningful results. Considerations for selecting appropriate comparators include:
- Relevance: The comparator should be relevant to the research question and reflect standard clinical practice.
- Equivalence: The comparator should be well-established and known to have a specific effect, making it suitable for comparison.
- Generalizability: The chosen comparator should be applicable to the target patient population and reflect real-world conditions.
By selecting appropriate comparators, researchers can strengthen the validity of the trial results and provide valuable insights into the intervention’s performance compared to existing treatments.
The Ethical Debate Surrounding Placebo Controls
The use of placebos in RCTs has been a subject of ethical debate. While placebos can provide valuable information and contribute to the advancement of medical knowledge, the ethical implications of withholding potentially beneficial treatments from participants should not be underestimated. Ethical considerations are essential in trial design and participant protection.
To address these ethical concerns, researchers must carefully weigh the risks and benefits, consider alternative study designs, use appropriate controls, and ensure informed consent and participant well-being throughout the trial. Ethical review boards play a crucial role in evaluating the justification and appropriateness of placebo-controlled trials to ensure that participant rights and safety are upheld.
| Advantages of Placebo-Controlled Trials | Limitations of Placebo-Controlled Trials |
|---|---|
| Eliminates bias | Ethical concerns regarding placebo use |
| Evaluates true treatment effect | Potential denial of potentially beneficial treatments |
| Minimizes confounding variables |
Double-Blind Trials: A Gold Standard for Objective Evidence
When it comes to providing objective evidence in randomized controlled trials (RCTs), double-blind trials are widely regarded as the gold standard. In these trials, neither the participants nor the researchers involved in the study know which intervention the participant is receiving. This blinding process effectively minimizes bias and enhances the validity of the study results.
The use of blinding techniques ensures that the allocation of interventions does not influence assessment or data collection. By keeping both participants and researchers unaware of the specific intervention being administered, the potential for bias is greatly reduced. This blinding method leads to more reliable and unbiased evidence, making double-blind trials highly valued in the scientific community.
By implementing double-blind trials, researchers can ensure that the study outcomes are not influenced by participant bias or researcher expectations. Through the rigorous application of blinding, double-blind trials strengthen the validity of the evidence produced, providing a solid foundation for informed decision-making in clinical practice.
To learn more about the significance and benefits of double-blind trials in RCTs, refer to this study published on the National Center for Biotechnology Information website.
How www.editverse.com can help to publish exceptional studies?
At www.editverse.com, we offer comprehensive publication support services to researchers and authors, aiming to enhance the quality and impact of their RCT studies. Our team of experts provides valuable assistance in manuscript editing, formatting, and ensuring adherence to journal guidelines. By partnering with www.editverse.com, researchers can trust us to handle the technical aspects of their study, allowing them to focus on their research and disseminating their findings to the intended audience.
With our manuscript editing services, we meticulously review and polish the language, grammar, and structure of the research manuscript. Our editors are trained in scientific writing and can optimize the clarity, coherence, and conciseness of the text, ensuring that the research is effectively communicated to readers.
Formatting the manuscript according to journal guidelines is crucial for successful publication. Our team is well-versed in the formatting requirements of various journals and can ensure that the manuscript adheres to the specific style, citation, and reference guidelines. By aligning the manuscript with the journal’s formatting expectations, we increase the chances of acceptance and streamline the publication process.
Research dissemination plays a vital role in the impact of a study. Through our publication support services, we assist researchers in effectively sharing their findings with the scientific community and beyond. We can help authors develop engaging abstracts, create impactful graphical elements, and craft clear and concise summaries of their research. By effectively disseminating the study, researchers have the opportunity to contribute to the advancement of scientific knowledge and make a lasting impact in their field.
Partnering with www.editverse.com for publication support not only ensures that researchers meet the high standards of academic publishing but also saves them time and effort, allowing them to focus on their core research activities. Our goal is to support researchers in their scientific journey by providing exceptional publication services that enhance the visibility, reach, and impact of their studies.
Your manuscript writing partner: www.editverse.com
Conclusion
In conclusion, Randomized Controlled Trials (RCTs) are a vital research methodology for evaluating the efficacy and effectiveness of interventions in clinical research. The foundations of RCT methodology, including the significance of randomization, strengthening internal validity, and designing meaningful control groups, are crucial for conducting robust and reliable studies.
By implementing high-quality data collection and analysis, selecting appropriate comparators, and considering ethical considerations, RCTs can provide objective evidence for evidence-based medicine. These rigorous studies help researchers and healthcare professionals make informed decisions about the best interventions for patient care.
To enhance the quality and impact of RCT studies, researchers can leverage the support and expertise of platforms like wwe.editverse.com. With their comprehensive publication support services, including manuscript editing and formatting, researchers can ensure their findings reach the intended audience and contribute to the advancement of scientific knowledge.
FAQ
What is the significance of randomization in clinical trials?
Randomization minimizes bias and increases the internal validity of a study by ensuring each participant has an equal chance of being assigned to different intervention groups, reducing the risk of confounding variables affecting the study outcomes.
What are the different RCT designs used in clinical trials?
RCTs can utilize parallel, crossover, and factorial designs to evaluate the effects of interventions and control groups. Each design has its advantages and limitations, and the choice depends on the research question and available resources.
How do randomized controlled trials strengthen internal validity?
RCTs strengthen internal validity through randomization and control of confounding variables. By randomly assigning participants to different groups, the impact of both known and unknown confounding factors can be minimized, increasing confidence in causal relationships between interventions and outcomes.
What is the difference between efficacy and effectiveness in RCTs?
Efficacy refers to the performance of an intervention under ideal and controlled circumstances, while effectiveness evaluates the intervention’s performance under real-world conditions.
How are patient allocation and the randomization process managed in RCTs?
Patient allocation is critical in ensuring a balanced distribution of participants across intervention and control groups. Randomization techniques such as coin flipping or computer-generated random numbers are commonly used to assign patients to different groups, with complex techniques like blocking or stratification enhancing the randomization process.
What are the critical elements of experimental study design in RCTs?
Experimental study design in RCTs includes the inclusion of a control group, blinding to minimize bias, and allocation concealment to prevent selection bias.
How can we build a robust study team for effective RCT execution?
Clinicians, researchers, and statisticians play vital roles in designing and conducting RCTs. Involving statisticians early in the research process helps in planning appropriate statistical analyses, and effective coordination and communication among research sites are essential for managing multi-center trials.
How are inclusion and exclusion criteria set in RCTs?
Inclusion and exclusion criteria are used to define the study population and ensure meaningful comparisons between intervention and control groups. Balancing generalizability and bias minimization is crucial when setting these criteria.
What are the strategies for ensuring high-quality data collection and analysis in RCTs?
Proper processes and protocols for data collection should be implemented to ensure data integrity and accuracy in RCTs. Data management and quality assurance measures are crucial for maintaining data integrity throughout the study, and appropriate statistical analysis techniques should be employed to generate valid findings.
How do we establish clear parameters for statistical analysis in RCTs?
Establishing predefined endpoints helps focus data analysis on specific outcomes of interest. Strategies for handling data and employing appropriate statistical techniques are crucial in avoiding bias and producing valid findings in RCTs.
What is the difference between placebo-controlled trials and trials using active comparators?
Placebo-controlled trials assess if the intervention has a specific effect beyond patient expectations and natural healing processes, while trials using active comparators help evaluate the superiority or non-inferiority of the intervention compared to an existing standard treatment.
Why are double-blind trials considered the gold standard in RCTs?
Double-blind trials minimize bias and increase the validity of study results by ensuring that neither the participants nor the researchers involved in the study know which intervention the participant is receiving.
How can wwe.editverse.com help with publishing exceptional studies?
wwe.editverse.com offers comprehensive publication support services, including manuscript editing, formatting, and adherence to journal guidelines, to enhance the quality and impact of RCT studies and ensure their findings reach the intended audience.