Imagine following a medical recommendation that seems authoritative, but leads to inconsistent care. How do we separate trustworthy guidance from flawed advice? This question lies at the heart of modern healthcare.
We define a robust approach to care as the deliberate integration of three core elements. These are the best available research, seasoned clinical expertise, and individual patient values. This triad ensures decisions are both scientifically sound and patient-centered.
Rigorous evaluation of clinical recommendations is not optional; it is essential. Poorly developed guidelines can directly harm patient safety and waste valuable resources. They create confusion instead of clarity for professionals.
To address this critical need, we turn to the AGREE II instrument. This framework is the internationally recognized standard for assessing the methodological quality of clinical practice guidelines. It provides a structured way to ensure recommendations are built on a solid foundation.
The tool’s design is comprehensive. It organizes 23 key items across six distinct domains. This structure allows for a systematic and thorough appraisal process.
Our purpose is to provide clear, actionable guidance. We will show researchers and healthcare professionals how to implement this critical appraisal framework effectively. Mastering this skill empowers you to confidently identify and utilize high-quality guidance in your work.
Key Takeaways
- High-quality clinical guidelines are vital for patient safety and effective care.
- A robust approach to care blends research evidence, clinical skill, and patient preferences.
- The AGREE II framework is the gold standard for evaluating guideline development quality.
- The instrument uses a structured method across multiple domains for assessment.
- Learning to apply this tool helps distinguish reliable recommendations from those with weaknesses.
- Systematic evaluation leads to more consistent and improved patient outcomes.
Introduction to Evidence Based Practice and the Role of AGREE II
Effective clinical practice demands a balanced integration of research findings, professional experience, and individual patient needs. This approach ensures decisions are scientifically sound while respecting personal circumstances.
Defining EBP: Best Evidence, Clinical Expertise, and Patient Values
Evidence-based practice combines three essential components. The first involves using current, high-quality research from systematic investigations. Clinical expertise represents the judgment skills professionals develop through experience.
Patient values ensure care aligns with personal preferences and cultural contexts. This triad forms the foundation for reliable clinical decisions.
PICO/PICOT Format and Evidence Hierarchies
The PICO framework provides a structured method for clinical questions. It addresses Population, Intervention, Comparison, and Outcome. The optional Time component creates the PICOT format for more specific queries.
Understanding evidence hierarchies is crucial for proper evaluation. Different study designs offer varying levels of reliability in establishing causality.
| Evidence Level | Study Type | Strength |
|---|---|---|
| Highest | Systematic Reviews | Strongest causal inference |
| High | Randomized Controlled Trials | Reduces bias effectively |
| Moderate | Cohort Studies | Good for prognosis |
| Lower | Case Reports | Limited generalizability |
Essential databases like PubMed and Cochrane Library provide access to these resources. The development of clinical guidelines follows specific methods to ensure quality. Proper implementation requires following established steps for successful outcomes.
AGREE II tool evidence based practice
Six distinct domains form the foundation of a comprehensive guideline assessment system. This structured approach allows for systematic evaluation of methodological quality. The instrument provides a reliable framework for professionals.
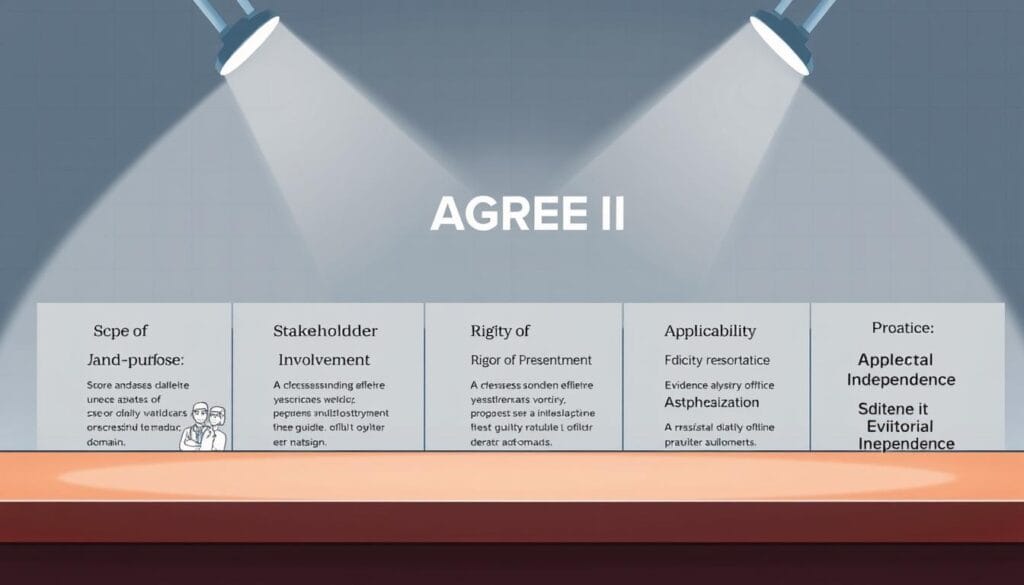
Overview of the AGREE II Instrument and Its Purpose
The assessment system contains 23 specific items organized across six key areas. Each item uses a 7-point scale for precise evaluation. Domain scores are calculated by summing individual item ratings.
This methodology was developed through international collaboration. The Next Steps Consortium enhanced the original framework with improved validity. Their work represents a significant advancement in guideline evaluation.
Understanding the Six Domains
The first domain examines scope and purpose. It assesses whether objectives and target populations are clearly defined. This ensures guidelines address appropriate health questions.
Stakeholder involvement represents the second critical area. It evaluates inclusion of diverse professional groups and patient perspectives. Comprehensive representation strengthens recommendation credibility.
Rigor of development forms the most extensive domain. It analyzes evidence selection methods and recommendation formulation processes. Systematic approaches minimize bias in final guidelines.
Clarity of presentation focuses on usability aspects. Recommendations must be specific and unambiguous for clinical application. Clear presentation supports effective implementation.
Applicability considers real-world implementation factors. It addresses barriers, resources, and monitoring criteria. Practical guidance enhances adoption success rates.
Editorial independence ensures objectivity throughout development. It examines funding influence and conflict of interest management. Transparency builds trust in final recommendations.
Validation studies published in the Canadian Medical Association Journal confirm the instrument’s reliability. McMaster University maintains current resources for users worldwide. This support system promotes consistent application across healthcare settings.
Implementing EBP Models and Appraisal Tools in Clinical Guidelines
The selection of implementation frameworks significantly influences the success of evidence-based guideline adoption. We compare four foundational models that provide structured approaches for integrating research into clinical settings.
Comparing EBP Models: Iowa, Johns Hopkins, ACE Star, and Stetler
The Iowa Model uses problem-focused and knowledge-focused triggers to identify practice questions. This systematic approach makes it ideal for organizational implementation.
The Johns Hopkins Nursing EBP Model structures the process through Practice question, Evidence, and Translation phases. It seamlessly integrates appraisal instruments within its methodology.
The ACE Star Model conceptualizes knowledge transformation through five sequential points. Guideline evaluation becomes a critical step in the translation phase.
The Stetler Model outlines five phases from preparation to application. Each framework offers distinct advantages for different healthcare contexts.
Utilizing Appraisal Tools: CASP, GRADE, AGREE II, and JBI Checklists
Specialized instruments serve complementary roles in comprehensive evaluation. CASP provides checklists for various study designs available at casp-uk.net.
GRADE rates evidence quality and recommendation strength. The AGREE instrument specifically evaluates guideline development methodology across key domains.
JBI offers checklists for systematic reviews and other evidence syntheses. External organizations maintain comprehensive registries of these methods and tools.
Practical implementation follows systematic steps with measurable outcomes. Organizations typically report 15-30% improvements in targeted safety metrics and cost savings.
Conclusion
Mastering the systematic evaluation of clinical recommendations is a fundamental skill for advancing healthcare quality. The comprehensive framework we’ve discussed, with its six core domains, provides a robust methodology for this critical task.
This structured approach ensures that adopted guidance demonstrates methodological rigor, transparency, and freedom from bias. Its reliability is well-documented, as shown in studies like the one published on the instrument’s reliability and internal consistency.
We encourage you to take immediate, practical steps. Identify a relevant guideline and assemble a small appraisal team. Free training resources are readily available to build your competence.
The return on this investment is substantial. Organizations report significant improvements in patient safety and cost savings. This process is a cornerstone of sound clinical decision-making, complementing other critical skills like navigating guidelines for systematic reviews.
Your commitment to this standard directly contributes to safer, more effective, and consistent patient care. Begin your first evaluation today.
What is the primary purpose of the AGREE II instrument?
The AGREE II instrument is a method tool designed to assess the quality and reporting of clinical practice guidelines. Its main purpose is to provide a structured framework for evaluating the development process, ensuring guidelines are trustworthy and based on robust methodology. This helps clinicians, policymakers, and researchers identify high-quality recommendations for evidence based practice.
How many domains are assessed in the AGREE II tool, and what do they measure?
The AGREE II tool evaluates guidelines across six distinct domains. These areas include scope and purpose, stakeholder involvement, rigour of development, clarity of presentation, applicability, and editorial independence. Each domain contains multiple items that collectively assess the validity and potential usefulness of a clinical guideline.
Who developed the AGREE II tool, and is it widely accepted?
The AGREE Next Steps Consortium, which includes researchers from McMaster University and the Canadian Medical Association, developed this instrument. It is internationally recognized and published in the Canadian Medical Association Journal. Its widespread adoption by external organizations underscores its credibility in the guideline development community.
How is an overall AGREE II score calculated?
An overall score is not calculated by simply summing domain scores. Instead, appraisers provide separate quality ratings for each of the six domains. Users then make an overall judgment about the guideline’s quality based on these domain assessments. This method allows for a nuanced evaluation of a guideline’s strengths and areas for improvement.
Where can I find resources to support the application of AGREE II?
The official AGREE Enterprise website offers a comprehensive toolkit, including the user manual, training resources, and links to relevant publications. These resources are essential for correctly applying the instrument’s assessment criteria and ensuring consistent, valid appraisals of clinical guidelines.